Help Your Patients Start and Stay on STELARA®
- Benefits Investigation & Prescription Enrollment Form - GastroenterologyA way to find out if STELARA® is covered by the patient's insurance plan, including requirements for coverage or prior authorization, any out-of-pocket costs, and approved pharmacies.
Benefits Investigation & Prescription Enrollment Form - Gastroenterology (en español para Puerto Rico) - Benefits Investigation & Prescription Enrollment Form - Gastroenterology (en español para Puerto Rico)A way to find out if STELARA® is covered by the patient's insurance plan, including requirements for coverage or prior authorization, any out-of-pocket costs, and approved pharmacies. En español.
- Benefits Investigation & Prescription Form - Dermatology & RheumatologyA way to find out if STELARA® is covered by the patient's insurance plan, including requirements for coverage or prior authorization, any out-of-pocket costs, and approved pharmacies.
Benefits Investigation & Prescription Form - Dermatology & Rheumatology (en español para Puerto Rico) - Benefits Investigation & Prescription Form - Dermatology & Rheumatology (en español para Puerto Rico)A way to find out if STELARA® is covered by the patient's insurance plan, including requirements for coverage or prior authorization, any out-of-pocket costs, and approved pharmacies. En español.
- Business Associate AgreementComplete a Business Associate Agreement for your practice only once. No individual patient authorizations are required.
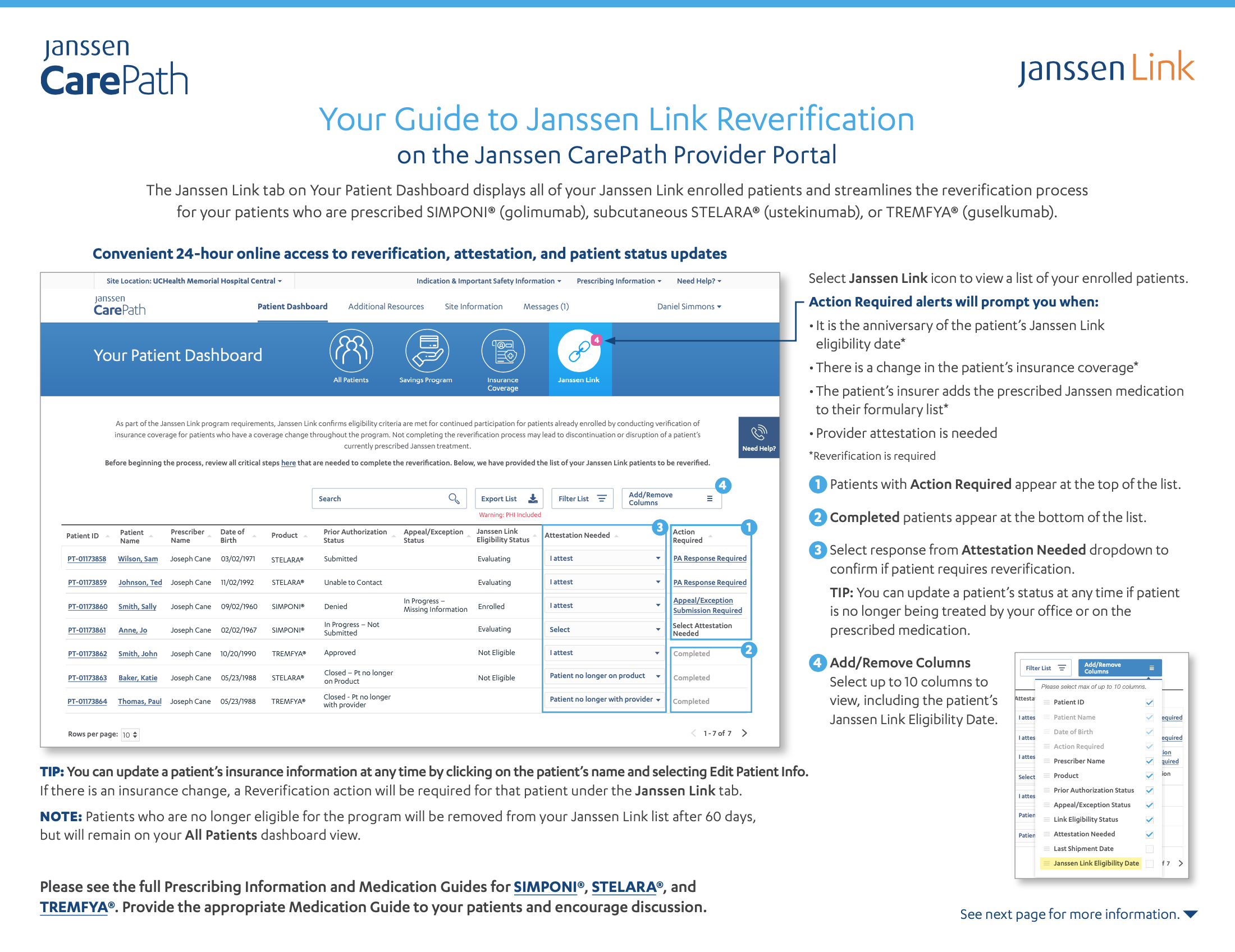
- Delay & Denial Support Reverification GuideUse this guide to learn how to confirm your patient's eligibility for Delay & Denial Support/Janssen Link if they have previously been eligible.
- Letter of Exception (Crohn’s Disease)A template that you can fill out and submit to a patient’s health insurance provider asking them to cover a medication that is not on formulary.
- Letter of Exception (Plaque Psoriasis)A template that you can fill out and submit to a patient’s health insurance provider asking them to cover a medication that is not on formulary.
- Letter of Exception (Psoriatic Arthritis)A template that you can fill out and submit to a patient’s health insurance provider asking them to cover a medication that is not on formulary.
- Letter of Exception (Ulcerative Colitis)A template that you can fill out and submit to a patient’s health insurance provider asking them to cover a medication that is not on formulary.
- Letter of Medical NecessityA template that you can fill out and submit to a patient’s health insurance provider. You may use it to explain why STELARA® is medically necessary for your patient.
- Patient Affordability OptionsDiscover options that can make STELARA® more affordable for your patients.
- Patient Authorization FormIndividual patient form for offices without a Business Associate Agreement.
Patient Authorization Form (en español) - Patient Authorization Form (en español)Individual patient form for offices without a Business Associate Agreement.
- Prescribing Information & Medication Guide (en español)Product information for STELARA®. En español.
- Resource GuideA comprehensive summary of support tools for your office to help patients start and stay on treatment.
- Savings Program Additional Co-pay Support Form
- Savings Program Assignment of Benefits FormA form the patient can submit that allows Janssen CarePath to reimburse the provider directly.
- Savings Program EOB Clarification FormUse this form when the Explanation of Benefits (EOB) statement does not indicate that the patient received STELARA®.
- Savings Program OverviewEligible patients using commercial or private insurance can save on out-of-pocket costs for STELARA®.
- Savings Program Patient Enrollment FormFax or mail this completed form to enroll your patient in the Savings Program for STELARA®.
- Savings Program Rebate FormA form the patient can submit if the pharmacy isn’t able to process the Savings Program card or for a medical benefit rebate.
- Savings Program Rebate Form - Accumulator MedicalA rebate form that when submitted along with the Explanation of Benefits (EOB) requests a rebate check to be sent directly to the patient.
- Savings Program Rebate Form - Accumulator PharmacyA form that is submitted when the pharmacy can't process the
STELARA withMe Savings Program card or Virtual Payment Card. - Specialty Distributors for IV Infusion
- Verification of Benefits Guide (Medical)A guide to understanding the Verification of Benefits for your patient’s medical benefits.
- Verification of Benefits Guide (Pharmacy)A guide to understanding the Verification of Benefits for your patient’s pharmacy benefits.

Help Your Patients Start and Stay on STELARA®
STELARA withMe for Your Patients
STELARA withMe provides a range of dedicated support and resources to help make it easier for patients as they begin, and continue, their STELARA® treatment journey.
STELARA withMe is all about dedicated support:
- Nurse Navigator
- Infusion Services
- Specialty Pharmacy Enhanced Services
- Reimbursement Support
- Access and Affordability Support
Use this form to learn more and enroll your patient.
Janssen Patient Account
Your patients and their caregivers can create an online account at MyJanssenCarePath.com where they can learn about their insurance coverage. They can also:
- Enroll in the STELARA withMe Savings Program
- Manage their Savings Program benefits
- Find support throughout their treatment journey
Specialty Distributors / Pharmacies
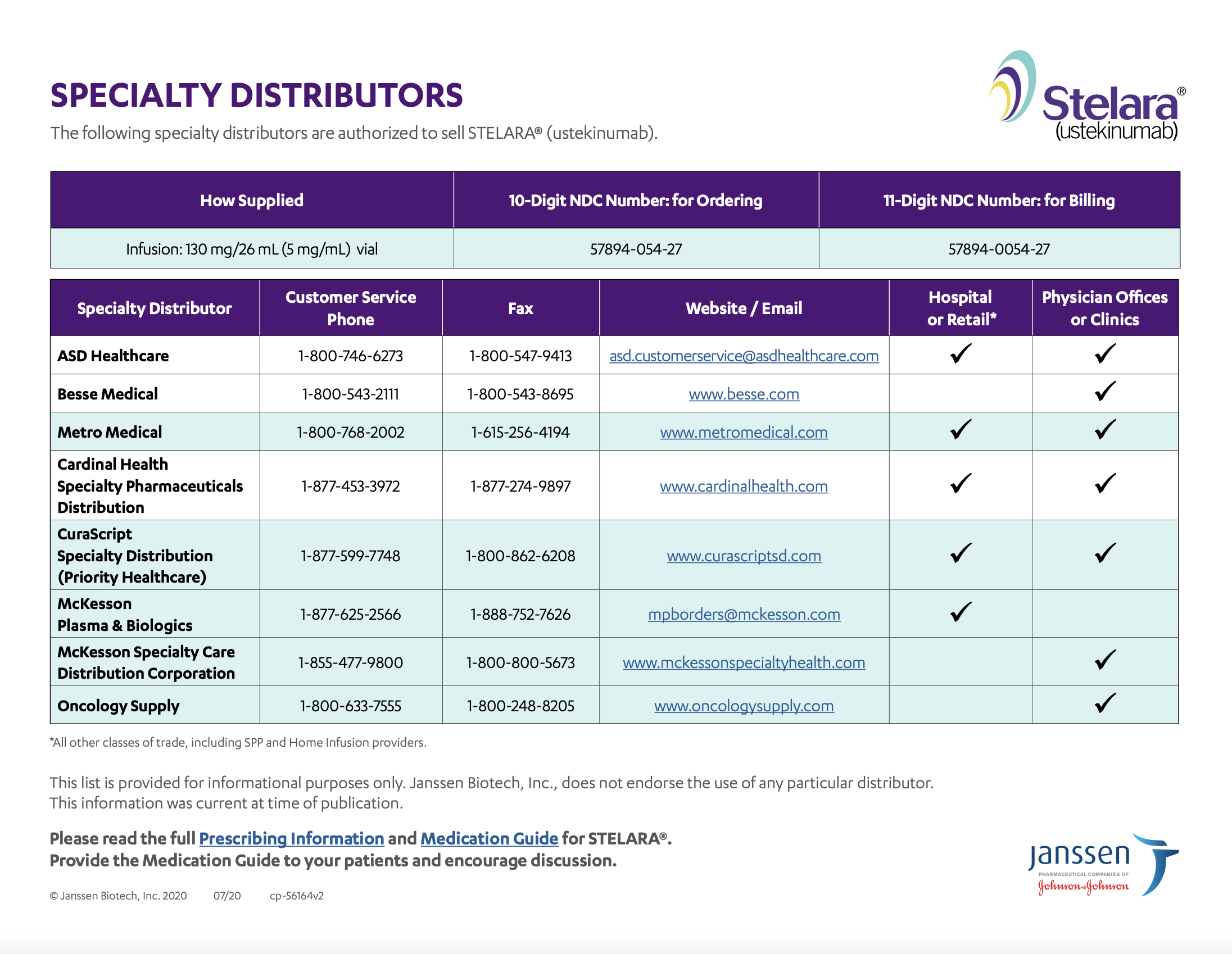
STELARA® is available from a wide range of both specialty distributors and pharmacies. Find a specialty distributor authorized to sell STELARA® vials for intravenous infusion directly to your office or hospital.
Your dedicated Care Coordinator can also provide:
- Identification of pharmacies based on your patient’s insurance coverage
- Prescription triage to pharmacy for coordination of medication delivery to your patient's home, your office, or other convenient location
To learn more, please call us at 844-4withMe (844-494-8463), Monday–Friday, 8:00 AM to 8:00 PM ET. Multilingual phone support is available.
Infusion Site Locator
Utilize the search capabilities of 2infuse.com to help you locate an infusion site convenient to your patient’s home or office. Searchable by city or ZIP code, this helpful site provides detailed search results organized by driving distance. This website also includes valuable information about the infusion sites such as business hours, insurance plans accepted, and medications infused.
Find an infusion center.
Are you an infusion center?
Register to list with 2infuse.com
Step-by-Step Injection Demonstration Videos
Your patients can watch these step-by-step injection demonstration videos to be reminded how to take their STELARA® medication.
- Share this STELARA® injection demonstration video with your patients who have active psoriatic arthritis or moderate to severe plaque psoriasis.
- Share this STELARA® injection training overview video with your patients who have moderately to severely active Crohn’s disease or moderately to severely active ulcerative colitis.
Janssen Nurse Support for Infusion Treatment
Even after you’ve spoken to your patient about infusion treatment with STELARA®, they may still have questions. For patients not enrolled in STELARA withMe, Janssen Nurse Support* can help answer their questions about the infusion process and provide more information about how to prepare for their infusion.
Connect your patients with Janssen Nurse Support at 877-CarePath (877-227-3728), available Monday–Friday, 9:00 AM to 8:00 PM ET. At all other times, a nurse will typically return your call in 15 minutes.
*Nurse Support is limited to education for patients about their Janssen therapy, its administration, and/or their disease. It is intended to supplement a patient's understanding of their therapy, and is not intended to provide medical advice, replace a treatment plan from the patient's doctor or nurse, provide case management services, or serve as a reason to prescribe.
Janssen Nurse Support for Injection Treatment
Even after you’ve trained your patients to give themselves an injection of STELARA®, they may still have questions. For patients not enrolled in STELARA withMe, Janssen Nurse Support* can help answer their questions about giving themselves an injection at home, preparing their injection site prior to self-injecting, and properly disposing of their used syringe.
Connect your patients with Janssen Nurse Support at 877-CarePath (877-227-3728), available Monday–Friday, 9:00 AM to 8:00 PM ET.
At all other times, a nurse will typically return their call in 15 minutes.
*Nurse Support is limited to education for patients about their Janssen therapy, its administration, and/or their disease. It is intended to supplement a patient's understanding of their therapy, and is not intended to provide medical advice, replace a treatment plan from the patient's doctor or nurse, provide case management services, or serve as a reason to prescribe.
Convenient Disposal of Used Devices
Patients who've received approval to inject at home and have been properly trained can use Safe Returns® as a simple, safe, and convenient way to dispose of their used devices—at no cost to the patient. When patients sign up, they'll receive a certified Safe Returns® sustainable mail-back envelope in the mail, along with easy-to-follow mail-back instructions. To sign up for Safe Returns®, patients can call 877-CarePath (877-227-3728) or fill out the enrollment form at JanssenSafeReturns.com/register.
Education and Tools
Additional education and tools for patients that can help in managing their treatment and condition can be found at Stelarainfo.com.
Internet Resources for Your Patients
|
There are many useful resources available online that may help your patients understand and manage their diseases and their treatment with STELARA®. You may find the websites listed here helpful as referrals in educating your patients about their condition. The links provided here are for informational purposes only. No endorsement or sponsorship is implied. |
STELARA® Patient Website
|
This website contains information about STELARA®. On this website, patients can get helpful information about their condition. |
American Academy of Dermatology
|
The American Academy of Dermatology is dedicated to providing dermatologists with the information and resources they need to serve their patients. Patients can learn more about dermatologists and the conditions they treat. |
National Psoriasis Foundation
|
The National Psoriasis Foundation (NPF) is a non-profit organization with a mission to drive efforts to cure psoriatic disease and improve the lives of those affected. |
Arthritis Foundation
|
The mission of the Arthritis Foundation is to improve lives through leadership in the prevention, control, and cure of arthritis and related diseases. This website includes education and online community resources for people with arthritis and their families. |
Arthritis Today
|
The online home of the Arthritis Foundation’s “Arthritis Today” magazine is a great source of tips and information for people living with arthritis. |
CreakyJoints
|
CreakyJoints provides treatment information, advice from professionals, and public forums for people living with arthritis to share their experiences. |
Crohn's & Colitis Foundation of America
|
The Crohn's & Colitis Foundation of America is a non-profit, volunteer-driven organization dedicated to finding the cure for Crohn's disease and ulcerative colitis. The website contains medical information, news, and events about digestive diseases. |
Call a Janssen CarePath Care Coordinator at 844-4withMe (844-494-8463), Monday−Friday, 8:00 AM to 8:00 PM ET. Multilingual phone support available.
Sign up or log in to the Provider Portal at JanssenCarePathPortal.com where you can request and review benefits investigations, enroll eligible patients in the STELARA withMe Savings Program, and view their Savings Program transactions.