ICD-10 Codes
- Janssen CarePath Oral PAH Savings ProgramEligible patients using commercial or private insurance can save on out-of-pocket-costs for UPTRAVI®.
- Patient Affordability OptionsDiscover options that can make UPTRAVI® more affordable for your patients.
- Patient Authorization FormA form for patients to allow their providers, insurers, and Janssen to share health information about them.
Patient Authorization Form (en español) - Patient Authorization Form (en español)A form for patients to allow their providers, insurers, and Janssen to share health information about them.
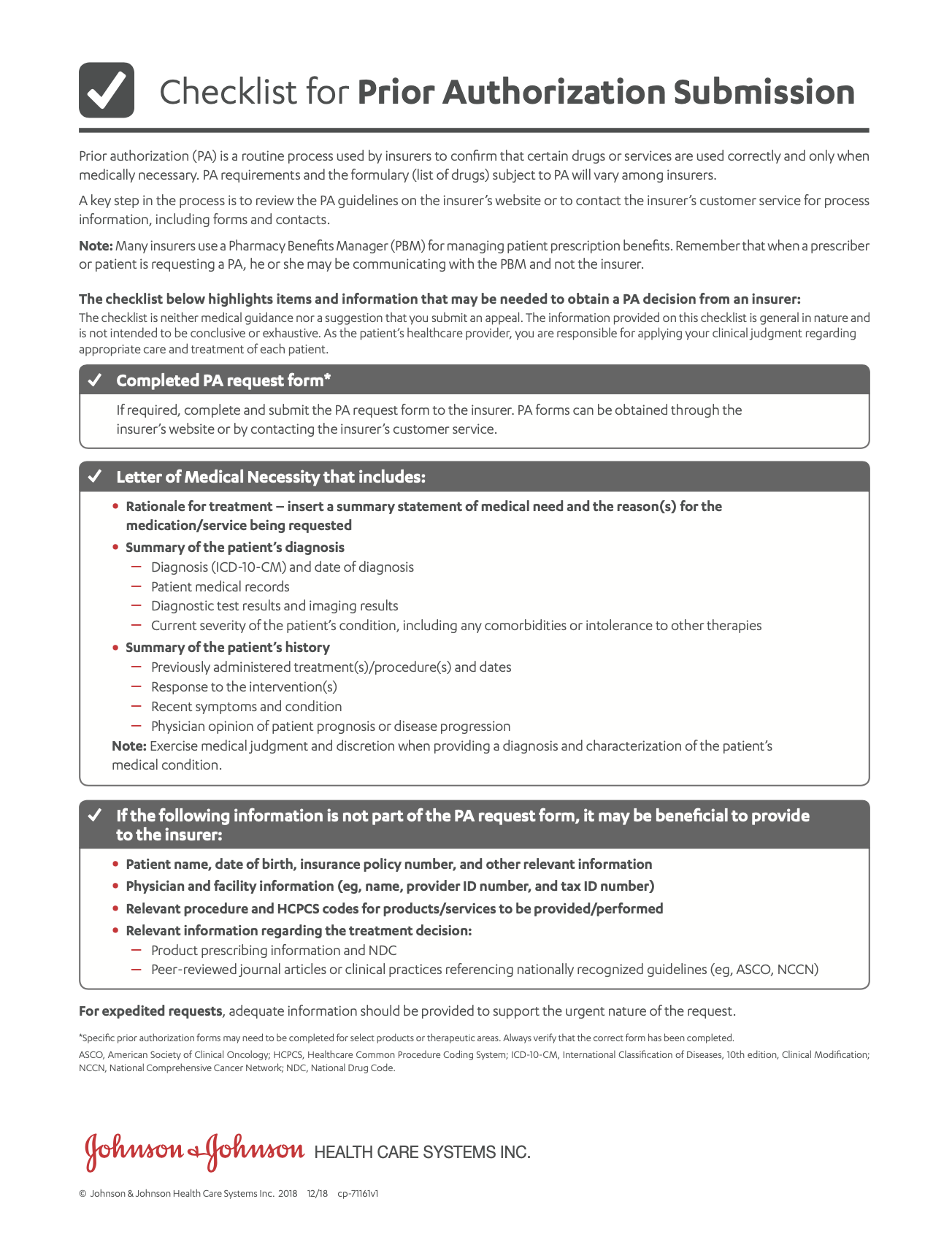
- Prior Authorization TipsA list of items and information that may be needed to obtain a coverage decision from an insurer.
- Sample Letter of AppealA template to send to patients' insurance companies to help get patients on treatment.
- Sample Statement of Medical NecessityA template that you can fill out and submit to a patient’s health insurance provider. You may use it to explain why UPTRAVI® is medically necessary for your patient.
- Steps to Getting Your MedicineA patient-friendly guide to the verification and delivery process.
Steps to Getting Your Medicine (en español) - Steps to Getting Your Medicine (en español)A patient-friendly guide to the verification and delivery process.
- UPTRAVI® Dose Adjustment Phase GuideA patient guide that highlights the important steps and information about titration with UPTRAVI®.
- UPTRAVI® Enrollment and Prescription FormComplete and submit this form first to get your patient started on UPTRAVI®.
- UPTRAVI® Enrollment and Prescription Form—For Veterans Affairs Patients OnlyComplete and submit this form first to get your Veterans Affairs patient started on UPTRAVI®.

ICD-10 Codes
Pulmonary Arterial Hypertension
| ICD-10 Indication | ICD-10 Code |
| Primary pulmonary hypertension | I27.0 |
|
|
|
|
|
|
| Pulmonary hypertension, unspecified | I27.20 |
| Secondary pulmonary arterial hypertension | I27.21 |
Collected in 10/21 and may change.
This information is not a promise of coverage or payment. It is not intended to give reimbursement advice or increase reimbursement by any payer. Legal requirements and plan information can be updated frequently. Contact the plan for more information about current coverage, reimbursement policies, restrictions, or requirements that may apply.
For more information on ICD-10, visit the CMS website.
SOURCE
ICD-10-CM 2022: The Complete Official Codebook. American Medical Association, 2021.