Delay and Denial Support
- Benefits Investigation & Prescription Form (Gastroenterology)A way to find out if SIMPONI® is covered by the patient's insurance plan, including requirements for coverage or prior authorization, any out-of-pocket costs, and approved pharmacies.
- Benefits Investigation & Prescription Form (Rheumatology)A way to find out if SIMPONI® is covered by the patient's insurance plan, including requirements for coverage or prior authorization, any out-of-pocket costs, and approved pharmacies.
Benefits Investigation & Prescription Form – en español para Puerto Rico (Reumatólogo) - Benefits Investigation & Prescription Form – en español para Puerto Rico (Reumatólogo)If you choose not to use the Provider Portal, complete this form and fax it back to us.
- Business Associate AgreementComplete a Business Associate Agreement for your practice only once. No individual patient authorizations are required.
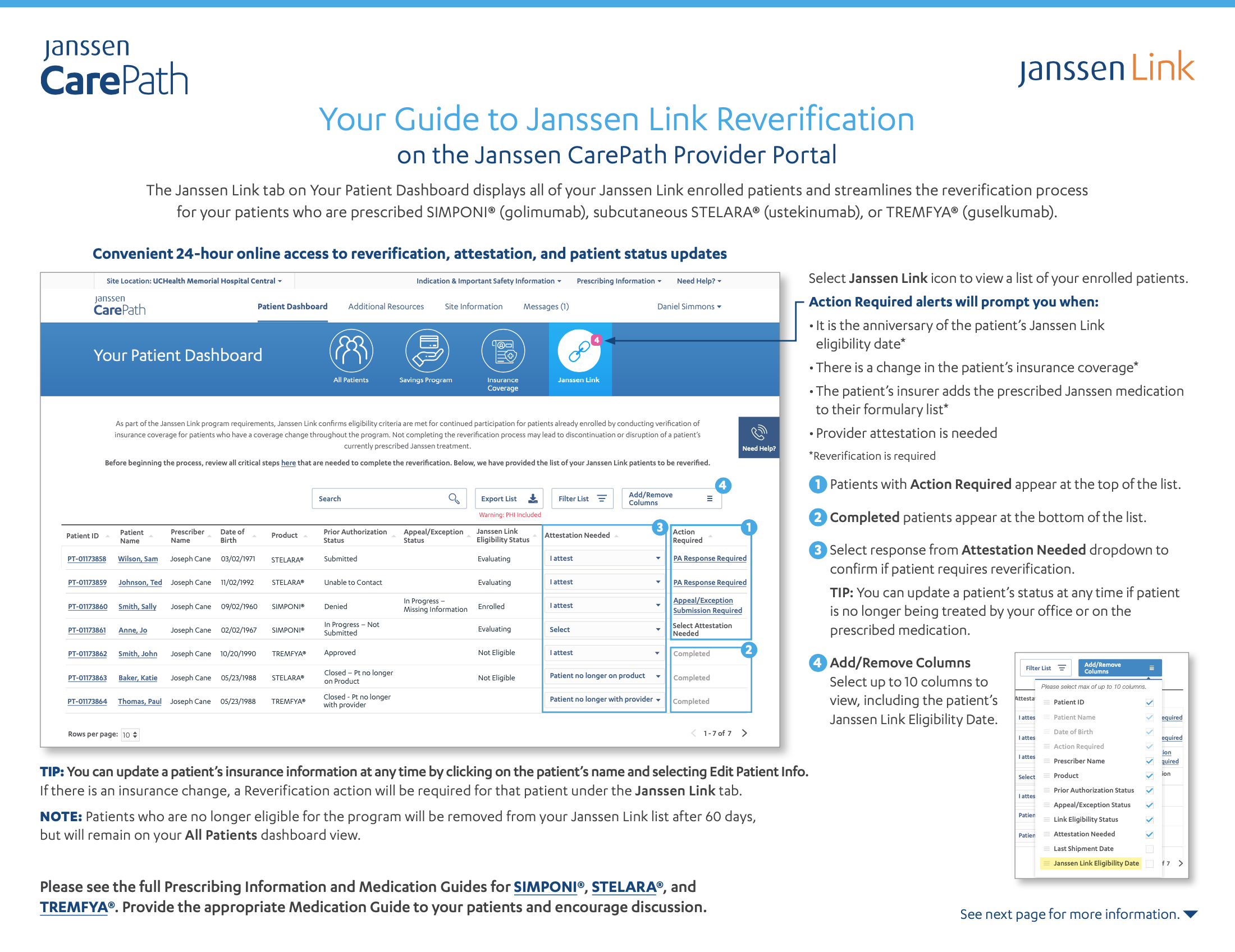
- Delay & Denial Support Reverification GuideUse this guide to learn how to confirm your patient's eligibility for Delay & Denial Support/Janssen Link if they have previously been eligible.
- Janssen CarePath Resource GuideA comprehensive summary of support tools for your office to help patients start and stay on treatment.
- Letter of Exception (Rheumatology)A template that you can fill out and submit to a patient’s health insurance provider asking them to cover a medication that is not on formulary.
- Letter of Exception (Ulcerative Colitis)A template that you can fill out and submit to a patient’s health insurance provider asking them to cover a medication that is not on formulary.
- Letter of Medical NecessityA template that you can fill out and submit to a patient’s health insurance provider. You may use it to explain why SIMPONI® is medically necessary for your patient.
- Patient Account Overview
- Patient Affordability OptionsDiscover options that can make SIMPONI® more affordable for your patients.
- Patient Authorization FormIndividual patient form for offices without a Business Associate Agreement.
Patient Authorization Form (en español) - Patient Authorization Form (en español)Individual patient form for offices without a Business Associate Agreement.
- Prescribing Information & Medication Guide (en español)Product information for SIMPONI®. En español.
- Savings Program OverviewEligible patients using commercial or private insurance can save on out-of-pocket costs for SIMPONI®.
- Savings Program Rebate FormA form the patient can submit if the pharmacy isn’t able to process the Janssen CarePath Savings Program card.
- Verification of Benefits Guide (Medical)A guide to understanding the Verification of Benefits for your patient’s medical benefits.
- Verification of Benefits Guide (Pharmacy)A guide to understanding the Verification of Benefits for your patient’s pharmacy benefits.

Delay and Denial Support
Need Delay and Denial Support?
- Complete a benefits investigation request in the Provider Portal at JanssenCarePathPortal.com
- OR
-
Download the Benefits Investigation and Prescription Form (Gastroenterology, Rheumatology), complete the form, and fax to Janssen CarePath at 855-224-5072.
Program Requirements
To be eligible, patient must have:
- 1 A SIMPONI® prescription for an on-label, FDA-approved indication
- 2 Commercial insurance with biologics coverage
- 3 A delay of more than 5 business days or a denial of treatment from their insurance
In addition, for patient to be eligible, Prescriber must submit:
- 4 A program enrollment form*
- 5 A coverage determination form (ie, prior authorization or prior authorization with exception) to the commercial insurance
If coverage is denied, Prescriber must also submit a Letter of Formulary Exception, Letter of Medical Necessity or appeal within 90 days of patient becoming eligible for patient to stay in the program.
Patient is not eligible if:
- 1 Patient uses any state or federal government-funded healthcare program to cover medication costs
- (Examples of these programs are Medicare, Medicaid, TRICARE, Department of Defense, and Veterans Administration)
- 2 Prior authorization is denied due to missing information on coverage determination form, use for a non-FDA-approved indication or invalid clinical rationale
Patient is eligible until commercial insurance covers the medication. Program requires periodic verification of insurance coverage status to confirm continued eligibility.
Delay and Denial Support covers the cost of therapy only—not associated administration cost. Prescriber cannot bill commercial insurance plan for any part of the prescribed subcutaneous treatment. Patient cannot submit the value of the free product as a claim for payment to any health plan. Program good only in the United States and its territories. Void where prohibited, taxed, or limited by law. Program terms may change.
*Delay and Denial Support, via Janssen CarePath, cannot accept any information without an executed Business Associate Agreement or Patient Authorization on file. The patient authorization can be found on the Prescription Information and Enrollment Form. Or patient can create an account on MyJanssenCarePath.com and electronically sign a patient authorization there.
Participating prescribers authorize Janssen to:
- 1 Conduct a benefits investigation and confirm prior authorization requirements
- 2 Provide prior authorization form assistance and status monitoring, including the exceptions and appeals processes
- 3 Refer eligible patients to Wegmans Specialty Pharmacy for further program support and shipment of medication
- 4 Support the transition of patients to commercial product
- 5 Check insurance coverage status during the program